How to Get Rid of Battery Acid?
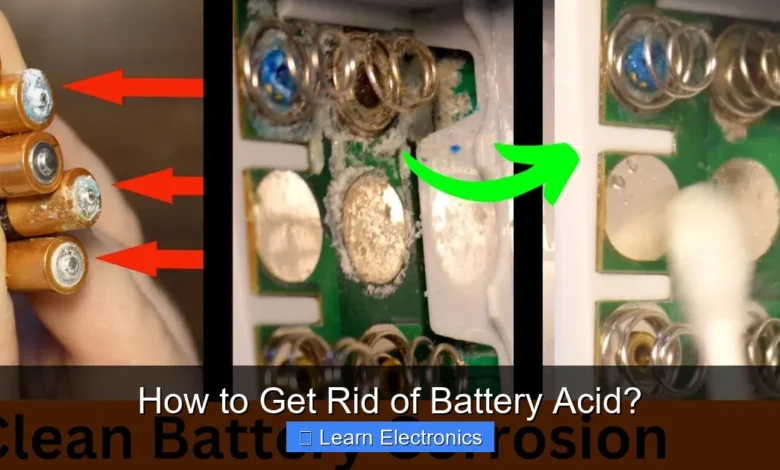
How to Get Rid of Battery Acid? involves a precise and safety-conscious approach of neutralization, careful cleanup, and responsible disposal. This critical process is essential to prevent severe personal injury, corrosive damage to property, and significant environmental contamination. Understanding this technique is paramount for anyone dealing with lead-acid batteries, from automotive enthusiasts to UPS system maintainers.
Quick Answers to Common Questions
What should I do immediately if I get battery acid on my skin?
Quickly rinse the affected area with large amounts of cool water for at least 15-20 minutes. Removing the battery acid quickly is key to minimizing irritation or burns.
How do I safely clean up a small battery acid spill?
First, protect yourself with gloves and eye protection. Then, carefully apply a generous amount of baking soda or a baking soda paste directly to the battery acid spill until it stops fizzing, which neutralizes it.
Can I just use water to neutralize battery acid on a surface?
While water is great for rinsing skin, it won’t effectively neutralize battery acid on a surface. You’ll need an alkaline substance, like baking soda, to safely counteract the acid and render it harmless.
📑 Table of Contents
Understanding Battery Acid and Its Hazards
Battery acid, primarily sulfuric acid (H₂SO₄) diluted with water, is a highly corrosive substance found in lead-acid batteries. These batteries are common in vehicles, motorcycles, uninterruptible power supplies (UPS), and various industrial applications. Even “maintenance-free” sealed lead-acid (SLA) batteries can sometimes leak, especially if overcharged or damaged.
The dangers associated with battery acid are manifold:
- Chemical Burns: Direct contact with skin or eyes can cause severe, deep chemical burns, leading to permanent tissue damage or blindness.
- Inhalation Hazards: Fumes, especially during charging or if the acid reacts with certain materials, can irritate the respiratory system.
- Corrosion: Sulfuric acid rapidly corrodes metals, eats through fabrics, and damages paint, concrete, and other materials.
- Environmental Impact: Improper disposal or spills can contaminate soil and water sources, harming ecosystems.
- Flammability/Explosion Risk: While the acid itself isn’t flammable, lead-acid batteries produce hydrogen gas during charging, which is highly explosive when mixed with air. A spark near a leaking battery can ignite hydrogen gas.
Recognizing these hazards underscores the importance of a robust plan for managing and neutralizing any acid spill, no matter how small.
Essential Safety Precautions
Before you even consider approaching a battery acid spill, prioritize safety. Adequate preparation and protective gear are non-negotiable.
Personal Protective Equipment (PPE)
- Eye Protection: Always wear chemical splash goggles or a full face shield. Regular eyeglasses are not sufficient.
- Hand Protection: Use acid-resistant gloves, such as those made from nitrile, neoprene, or PVC. Avoid latex gloves, as they may not offer adequate protection.
- Body Protection: Wear long sleeves, long pants, and chemical-resistant aprons or coveralls. Old clothes that can be discarded if contaminated are ideal.
- Foot Protection: Wear sturdy, closed-toe shoes, preferably chemical-resistant boots.
- Respiratory Protection: For larger spills or in poorly ventilated areas, consider a respirator to protect against fumes.
Workspace Preparation and First Aid
- Ventilation: Ensure the area is well-ventilated to disperse any fumes. If indoors, open windows and doors.
- Containment: Before beginning cleanup, try to contain the spill to prevent it from spreading further. Use absorbent materials like paper towels or an absorbent boom.
- First Aid Station: Have immediate access to a source of running water (eyewash station or a hose) for flushing skin or eyes in case of contact. A paste of baking soda and water can be applied to skin after flushing, but flushing with water is the first and most critical step.
- Material Readiness: Gather all necessary cleanup materials (neutralizer, brushes, scoops, disposal containers) before starting.
Never rush the process or compromise on safety. A moment of carelessness can lead to serious injury.
How to Get Rid of Battery Acid? Neutralization Techniques
Neutralization is the primary method for dealing with battery acid. It involves adding a base to the acid to bring its pH closer to neutral (pH 7), turning the dangerous acid into a less harmful salt and water solution.
Choosing Your Neutralizer
Several common household and industrial substances can be used to neutralize battery acid:
- Baking Soda (Sodium Bicarbonate – NaHCO₃): This is the most common and safest choice for small to moderate spills. It’s readily available, inexpensive, and its reaction with sulfuric acid produces harmless carbon dioxide gas, water, and sodium sulfate.
- Soda Ash (Sodium Carbonate – Na₂CO₃): Similar to baking soda but more potent. It’s often used for larger spills or in industrial settings.
- Hydrated Lime (Calcium Hydroxide – Ca(OH)₂): Also known as slaked lime, it’s a strong base suitable for very large spills. It reacts to form gypsum (calcium sulfate).
- Ammonia (NH₃ – diluted): While effective, ammonia produces strong fumes and should only be used in very well-ventilated areas. It’s generally less preferred for general spills due to its respiratory hazards.
For most household and automotive battery spills, baking soda is the recommended option due to its safety and availability.
Step-by-Step Neutralization
- Contain the Spill: If the acid is spreading, create a barrier around it using absorbent materials or even sand to prevent further spread.
- Apply the Neutralizer: Sprinkle a generous amount of your chosen neutralizer (e.g., baking soda) directly onto the spill. Start from the outside edges and work your way in. Apply enough to fully cover the acid.
- Observe the Reaction: You should observe fizzing or bubbling as the acid reacts with the base, releasing carbon dioxide gas. This indicates the neutralization process is underway.
- Stir and Wait: Gently stir the mixture with a non-metallic stick or brush to ensure complete contact. Add more neutralizer if fizzing continues vigorously or if the solution appears very liquid. Allow the reaction to subside.
- Rinse and Repeat (Optional but Recommended): Once the fizzing stops and the mixture forms a slurry or paste, you can carefully spray a small amount of water onto it. If it fizzes again, add more neutralizer. Continue until no further reaction occurs.
- Test pH (Optional for small spills, Recommended for larger ones): For larger or more critical spills, use pH paper or a pH meter to confirm the solution is neutral (pH 6-8). If it’s still acidic (below 6), add more neutralizer. If it becomes too alkaline (above 8), you can add a tiny amount of very diluted vinegar to balance it, but generally, slight alkalinity is safer than acidity.
The goal is to transform the hazardous liquid acid into a safer, more manageable solid or semi-solid form.
Cleanup and Disposal of Neutralized Waste
Once the battery acid has been neutralized, the next crucial steps involve collecting the resulting waste and disposing of it responsibly. Even after neutralization, the slurry or contaminated materials should not be treated as regular trash.
Collecting the Slurry
- Small Spills: For small, fully neutralized spills, use paper towels or absorbent rags to blot up the paste. Place these directly into a sturdy plastic bag.
- Larger Spills: Use a non-metallic scoop, shovel, or squeegee to collect the slurry. Kitty litter (clay-based, non-clumping) can also be used as an absorbent material, then swept up. Place all collected material into a heavy-duty, chemical-resistant plastic container or bag. Double-bagging is a good practice.
- Cleaning Surfaces: After removing the bulk of the neutralized material, wash the affected surface with soap and water multiple times, rinsing thoroughly. Check for any discoloration or remaining residue.
How to Get Rid of Battery Acid? Proper Disposal Practices
Even neutralized battery acid waste contains heavy metals (like lead from battery plates) and other chemicals that are harmful to the environment. Therefore, proper disposal is critical.
- Do NOT Pour Down Drains: Never pour neutralized or unneutralized battery acid, or the rinse water, down a drain unless explicitly permitted by local regulations for very small, perfectly neutral spills. Many jurisdictions prohibit this due to the potential for heavy metal contamination.
- Hazardous Waste Facilities: The safest and most environmentally responsible method for disposing of neutralized battery acid waste (slurry, contaminated absorbents, used PPE) is to take it to a designated household hazardous waste (HHW) collection facility. These facilities are equipped to handle and process such materials safely.
- Automotive Shops/Recycling Centers: Some automotive repair shops or battery recycling centers may accept contaminated cleanup materials, especially if you’re also recycling the old battery. Always call ahead to confirm their acceptance policies.
- Labeling: Clearly label the container with the neutralized waste (e.g., “Neutralized Battery Acid Waste – Contains Lead”) before transporting it.
Check with your local waste management authority, municipality, or environmental protection agency for specific guidelines and approved disposal sites in your area. Regulations vary significantly by location.
Dealing with Different Types of Battery Acid Spills
The approach to cleanup can vary slightly depending on where the spill occurs and the type of battery involved.
Car Battery Spills
Car batteries are a common source of sulfuric acid leaks. Spills can occur in the engine bay, on garage floors (concrete, asphalt), or even on vehicle carpet or upholstery.
- Engine Bay: Immediately neutralize the acid. Rinse thoroughly with water, being mindful of electrical components. Corrosion inhibitors may be applied afterward.
- Concrete/Asphalt: Neutralize as described. The acid can eat into concrete, leaving stains and weakening the material. Rinse and scrub thoroughly.
- Carpet/Upholstery: Act quickly. Neutralize the area liberally with baking soda, blot, then rinse with water and blot dry repeatedly. Professional cleaning may be required to remove stains and odors. Acid can permanently damage fibers.
Household Battery Leaks
It’s important to distinguish between lead-acid battery leaks (e.g., from small UPS units, sealed lead-acid alarms) and common household alkaline battery leaks (AA, AAA, C, D). Alkaline leaks are typically potassium hydroxide and require neutralization with a weak acid like vinegar or lemon juice, not baking soda. However, if it’s a lead-acid battery in a household setting, the neutralization process is the same as described above, just on a smaller scale.
For small sealed lead-acid battery leaks, follow the same safety and neutralization steps, using baking soda and proper disposal.
Battery Acid on Skin or Clothing
Immediate action is critical for personal exposure:
- Skin Contact: Immediately flush the affected area with copious amounts of running water for at least 15-20 minutes. Do not rub. After flushing, a paste of baking soda and water can be applied to further neutralize any remaining acid. Seek medical attention.
- Eye Contact: Flush eyes immediately with large amounts of running water for at least 20-30 minutes, holding eyelids open. Seek immediate emergency medical attention, even if symptoms seem mild.
- Clothing Contact: Carefully remove contaminated clothing while flushing skin. Rinse clothing thoroughly with water, then soak in a baking soda solution to neutralize any remaining acid before washing. Note that acid may have already damaged the fabric.
Prevention and Maintenance
The best way to deal with battery acid is to prevent spills from happening in the first place.
- Regular Inspection: Periodically check batteries for signs of leakage, corrosion around terminals, bulging cases, or cracks.
- Proper Charging: Overcharging can cause batteries to overheat, leak, and generate excessive hydrogen gas. Use a charger appropriate for your battery type and follow manufacturer guidelines.
- Ventilation During Charging: Always charge lead-acid batteries in a well-ventilated area to prevent the buildup of explosive hydrogen gas.
- Secure Storage: Store batteries in a cool, dry place away from direct sunlight and sources of ignition. Use battery boxes or trays to contain potential leaks and provide insulation.
- Avoid Overfilling: If your battery has removable caps, only fill with distilled water to the recommended level. Overfilling can lead to electrolyte overflow during charging.
- Handle with Care: When moving or replacing batteries, handle them carefully to avoid dropping or puncturing the casing.
| Neutralizer | Composition | Primary Application | Key Considerations |
|---|---|---|---|
| Baking Soda | Sodium Bicarbonate (NaHCO₃) | Small to moderate household/automotive spills | Readily available, safe, produces CO₂ gas |
| Soda Ash | Sodium Carbonate (Na₂CO₃) | Larger spills, industrial use | More potent than baking soda, requires careful handling |
| Hydrated Lime | Calcium Hydroxide (Ca(OH)₂) | Very large industrial spills, concrete surfaces | Strong base, can be dusty, produces gypsum |
By implementing these preventive measures, you can significantly reduce the risk of battery acid spills and the need for reactive cleanup.
Conclusion
Effectively managing battery acid spills is a critical skill for anyone working with or maintaining lead-acid batteries. The process of neutralizing battery acid, followed by meticulous cleanup and responsible disposal, safeguards personal health, preserves property, and protects our environment. Always prioritize safety by wearing appropriate PPE and working in a well-ventilated area. With the right knowledge and tools, you can confidently address battery acid leaks and ensure that this hazardous substance is rendered harmless and disposed of correctly. Remember, prevention through proper battery maintenance and handling is always the best defense against spills.
Frequently Asked Questions
What should I do immediately if battery acid spills?
If battery acid spills, your immediate priority should be safety. Ensure proper ventilation, keep pets and children away from the area, and quickly put on personal protective gear like chemical-resistant gloves and eye protection.
How do you neutralize battery acid after a spill?
To effectively neutralize battery acid after a spill, use baking soda (sodium bicarbonate). Sprinkle a generous amount directly onto the acid until any bubbling stops, which indicates the acid is no longer active. You can then carefully sweep up the neutralized residue.
What household items can be used to clean up battery acid?
Baking soda is the most effective household item for neutralizing and cleaning up battery acid due to its alkaline properties. After neutralization, you can use soap and water to clean the affected surface thoroughly. Always ensure you wear protective gear during the entire cleanup process.
Is battery acid dangerous to pets or children?
Yes, battery acid is extremely dangerous and highly corrosive to pets and children. Direct contact can cause severe chemical burns to skin, eyes, and internal organs if ingested. Always store batteries and dispose of acid safely out of reach.
What protective gear is necessary when handling battery acid?
When handling battery acid, it is crucial to wear appropriate personal protective equipment (PPE). This includes chemical-resistant gloves (such as nitrile or neoprene), safety glasses or goggles to protect your eyes, and long sleeves/pants to cover your skin.
How should I properly dispose of battery acid?
For neutralized battery acid spills, the residue can often be safely disposed of with regular household waste, but always confirm with local waste management guidelines. For larger quantities of unneutralized acid or old battery electrolytes, contact your local hazardous waste facility or recycling center for specific disposal instructions.
As an Amazon Associate, I earn commission from qualifying purchases.



